-
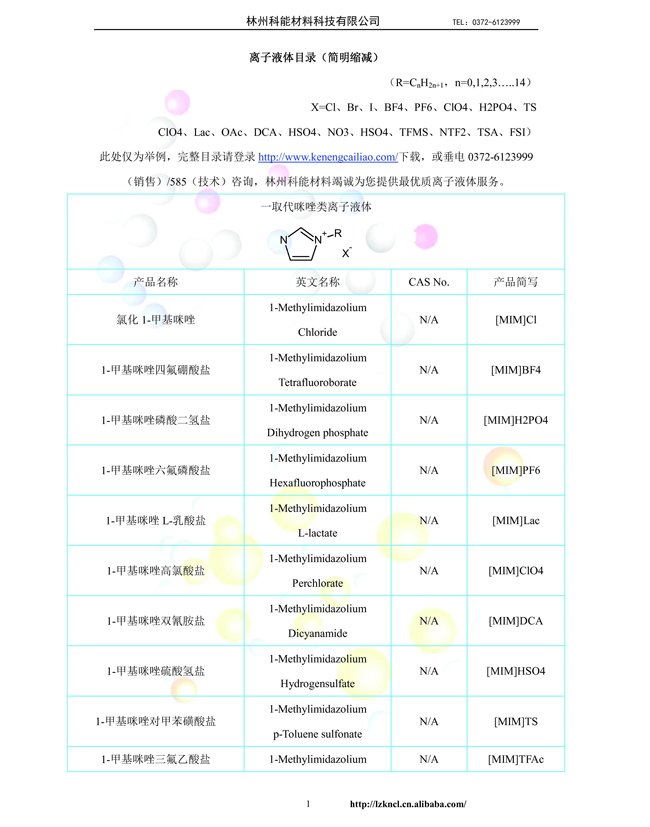 Catalogue of Ionic LiquidsCatalogue of Ionic Liquids2023-02-28
Catalogue of Ionic LiquidsCatalogue of Ionic Liquids2023-02-28 -
 Characteristics and advantages of ionic liquidsIonic liquids refer to liquids composed entirely of ions, such as KCI and KOH at high temperatures, which are in a liquid state and are considered ionic liquids. Substances composed of ions that are liquid at or near room temperature are called room temperature ionic liquids, room temperature molten salts, ionic liquids, etc. There is no unified name for them, but they tend to be abbreviated as ionic liquids.Ionic liquids are composed of positively charged ions and negatively charged ions, and they remain in a liquid state between negative 100 degrees Celsius and 200 degrees Celsius. In ionic compounds, the interaction force between anions and cations is Coulomb force, which is related to the charge quantity and radius of the anions and cations. The larger the ionic radius, the smaller the interaction force between them, and the lower the melting point of this ionic compound. Some ionic compounds have large volumes and loose structures of cations and anions, resulting in low interaction forces between them and melting points close to room temperature.Basic characteristics:(1) Wide liquid range, from below or near room temperature to above 300 degrees Celsius, with high thermal and chemical stability;(2) The vapor pressure is very low, non-volatile, and will not evaporate or dissipate during use and storage. It can be recycled, eliminating the environmental pollution problem of volatile organic compounds (VOCs),(3) High conductivity, large electrochemical window, and can be used as an electrolyte for electrochemical research of many substances;(4) The design of anions and cations can adjust their solubility in inorganic substances, water, substances, and polymers, and their acidity can be adjusted to superacid.(5) It has high polarity controllability, low viscosity, high density, and can form two-phase or multiphase systems, suitable for use as a separation solvent or as a new reaction separation coupling system;(6) It exhibits good solubility for a large number of inorganic and material substances, and has dual functions as a solvent and catalyst, making it suitable as a solvent or catalytic active carrier for many chemical reactions. Due to these special properties and behaviors of ionic liquids, they are considered to form three major green solvents together with supercritical CO2 and aqueous two-phase systems, and have broad application prospects.Related advantages:Ionic liquids are odorless and non flammable, with extremely low vapor pressure, making them suitable for use in high vacuum systems while reducing environmental pollution caused by volatilization;Ionic liquids have good solubility for both inorganic and inorganic substances, allowing reactions to occur under homogeneous conditions while reducing equipment volume;3. It has a wide operating temperature range (-40~300 ℃), good thermal and chemical stability, easy separation from other substances, and can be recycled;4. It exhibits the acidity of Lewis and Franklin acids, and the acid strength can be adjusted.2023-02-28
Characteristics and advantages of ionic liquidsIonic liquids refer to liquids composed entirely of ions, such as KCI and KOH at high temperatures, which are in a liquid state and are considered ionic liquids. Substances composed of ions that are liquid at or near room temperature are called room temperature ionic liquids, room temperature molten salts, ionic liquids, etc. There is no unified name for them, but they tend to be abbreviated as ionic liquids.Ionic liquids are composed of positively charged ions and negatively charged ions, and they remain in a liquid state between negative 100 degrees Celsius and 200 degrees Celsius. In ionic compounds, the interaction force between anions and cations is Coulomb force, which is related to the charge quantity and radius of the anions and cations. The larger the ionic radius, the smaller the interaction force between them, and the lower the melting point of this ionic compound. Some ionic compounds have large volumes and loose structures of cations and anions, resulting in low interaction forces between them and melting points close to room temperature.Basic characteristics:(1) Wide liquid range, from below or near room temperature to above 300 degrees Celsius, with high thermal and chemical stability;(2) The vapor pressure is very low, non-volatile, and will not evaporate or dissipate during use and storage. It can be recycled, eliminating the environmental pollution problem of volatile organic compounds (VOCs),(3) High conductivity, large electrochemical window, and can be used as an electrolyte for electrochemical research of many substances;(4) The design of anions and cations can adjust their solubility in inorganic substances, water, substances, and polymers, and their acidity can be adjusted to superacid.(5) It has high polarity controllability, low viscosity, high density, and can form two-phase or multiphase systems, suitable for use as a separation solvent or as a new reaction separation coupling system;(6) It exhibits good solubility for a large number of inorganic and material substances, and has dual functions as a solvent and catalyst, making it suitable as a solvent or catalytic active carrier for many chemical reactions. Due to these special properties and behaviors of ionic liquids, they are considered to form three major green solvents together with supercritical CO2 and aqueous two-phase systems, and have broad application prospects.Related advantages:Ionic liquids are odorless and non flammable, with extremely low vapor pressure, making them suitable for use in high vacuum systems while reducing environmental pollution caused by volatilization;Ionic liquids have good solubility for both inorganic and inorganic substances, allowing reactions to occur under homogeneous conditions while reducing equipment volume;3. It has a wide operating temperature range (-40~300 ℃), good thermal and chemical stability, easy separation from other substances, and can be recycled;4. It exhibits the acidity of Lewis and Franklin acids, and the acid strength can be adjusted.2023-02-28 -
 The function and precautions of electrolyteElectrolyte actionElectrolyte is the medium used in chemical batteries, electrolytic capacitors, etc. (with certain corrosiveness), providing ions for their normal operation. And ensure that the chemical reactions that occur during work are reversible.There are many benefits to using electrolytes as cathodes. Firstly, the large contact area between the liquid and the medium is helpful in increasing the capacitance. Secondly, electrolytic capacitors made with electrolytes can withstand high temperatures up to 260 degrees Celsius, which can be achieved through wave soldering (an important process in SMT surface mount installation), and also have strong voltage resistance.In addition, electrolytic capacitors using electrolytes as cathodes can self heal as long as the breakdown current is not sustained after the dielectric is broken down. But electrolytes also have their shortcomings. Firstly, it is prone to volatilization and leakage in high temperature environments, which has a significant impact on its lifespan and stability. Under high temperature and pressure, the electrolyte may also vaporize instantly, increasing in volume and causing explosions (commonly known as slurry explosions); The second is the ion conduction method used in the electrolyte, which has a very low conductivity of only 0.01S (conductivity, reciprocal of ohms)/CM, resulting in a particularly high ESR value (equivalent series resistance) of the capacitorusage methodThe original solution is used, with a lead plate as the cathode (negative electrode) and the workpiece as the anode (positive electrode), at 60-65 degrees Celsius, with a current density of 10-25 amperes per square decimeter, a voltage of 8-10 volts, and a duration of 5-8 minutes.matters needing attention1. The polishing solution will produce foam when it is used for electrolytic polishing at the initial stage, so the distance between the liquid level of the polishing solution and the top of the polishing tank should not be less than 15cm.2. Before entering the polishing tank, the residual moisture on the surface of stainless steel workpieces should be removed as much as possible. Excessive moisture carried by the workpiece may cause serious pitting on the polishing surface, local erosion, and result in the workpiece being scrapped.During the electrolytic polishing process, the stainless steel workpiece serving as the anode continuously transforms its iron and chromium elements into metal ions that dissolve into the polishing solution instead of depositing on the cathode surface. As the polishing process progresses, the concentration of metal ions continues to increase. When it reaches a certain value, these metal ions continuously precipitate from the polishing solution in the form of phosphates and sulfates, settling at the bottom of the polishing tank. For this reason, the polishing solution must be filtered regularly to remove these solid precipitates.4. During the operation of the polishing tank, water is lost due to evaporation and electrolysis, except for the continuous consumption of phosphoric acid and sulfuric acid. In addition, high viscosity polishing solution is constantly carried and lost by the workpiece, and the polishing solution level keeps decreasing. It is necessary to regularly add fresh polishing solution and water to the polishing tank5. Neutralized emissions meet current environmental requirements.6. This product is corrosive. Do not enter the eyes or mouth, and do not touch the skin. If accidentally touched, rinse immediately with clean water. In severe cases, seek medical attention for burns caused by strong acid;7. Store in a sealed and cool place for long-term effectiveness2023-02-28
The function and precautions of electrolyteElectrolyte actionElectrolyte is the medium used in chemical batteries, electrolytic capacitors, etc. (with certain corrosiveness), providing ions for their normal operation. And ensure that the chemical reactions that occur during work are reversible.There are many benefits to using electrolytes as cathodes. Firstly, the large contact area between the liquid and the medium is helpful in increasing the capacitance. Secondly, electrolytic capacitors made with electrolytes can withstand high temperatures up to 260 degrees Celsius, which can be achieved through wave soldering (an important process in SMT surface mount installation), and also have strong voltage resistance.In addition, electrolytic capacitors using electrolytes as cathodes can self heal as long as the breakdown current is not sustained after the dielectric is broken down. But electrolytes also have their shortcomings. Firstly, it is prone to volatilization and leakage in high temperature environments, which has a significant impact on its lifespan and stability. Under high temperature and pressure, the electrolyte may also vaporize instantly, increasing in volume and causing explosions (commonly known as slurry explosions); The second is the ion conduction method used in the electrolyte, which has a very low conductivity of only 0.01S (conductivity, reciprocal of ohms)/CM, resulting in a particularly high ESR value (equivalent series resistance) of the capacitorusage methodThe original solution is used, with a lead plate as the cathode (negative electrode) and the workpiece as the anode (positive electrode), at 60-65 degrees Celsius, with a current density of 10-25 amperes per square decimeter, a voltage of 8-10 volts, and a duration of 5-8 minutes.matters needing attention1. The polishing solution will produce foam when it is used for electrolytic polishing at the initial stage, so the distance between the liquid level of the polishing solution and the top of the polishing tank should not be less than 15cm.2. Before entering the polishing tank, the residual moisture on the surface of stainless steel workpieces should be removed as much as possible. Excessive moisture carried by the workpiece may cause serious pitting on the polishing surface, local erosion, and result in the workpiece being scrapped.During the electrolytic polishing process, the stainless steel workpiece serving as the anode continuously transforms its iron and chromium elements into metal ions that dissolve into the polishing solution instead of depositing on the cathode surface. As the polishing process progresses, the concentration of metal ions continues to increase. When it reaches a certain value, these metal ions continuously precipitate from the polishing solution in the form of phosphates and sulfates, settling at the bottom of the polishing tank. For this reason, the polishing solution must be filtered regularly to remove these solid precipitates.4. During the operation of the polishing tank, water is lost due to evaporation and electrolysis, except for the continuous consumption of phosphoric acid and sulfuric acid. In addition, high viscosity polishing solution is constantly carried and lost by the workpiece, and the polishing solution level keeps decreasing. It is necessary to regularly add fresh polishing solution and water to the polishing tank5. Neutralized emissions meet current environmental requirements.6. This product is corrosive. Do not enter the eyes or mouth, and do not touch the skin. If accidentally touched, rinse immediately with clean water. In severe cases, seek medical attention for burns caused by strong acid;7. Store in a sealed and cool place for long-term effectiveness2023-02-28


